IBM and Moderna have simulated the longest mRNA pattern without AI — they used a quantum computer instead

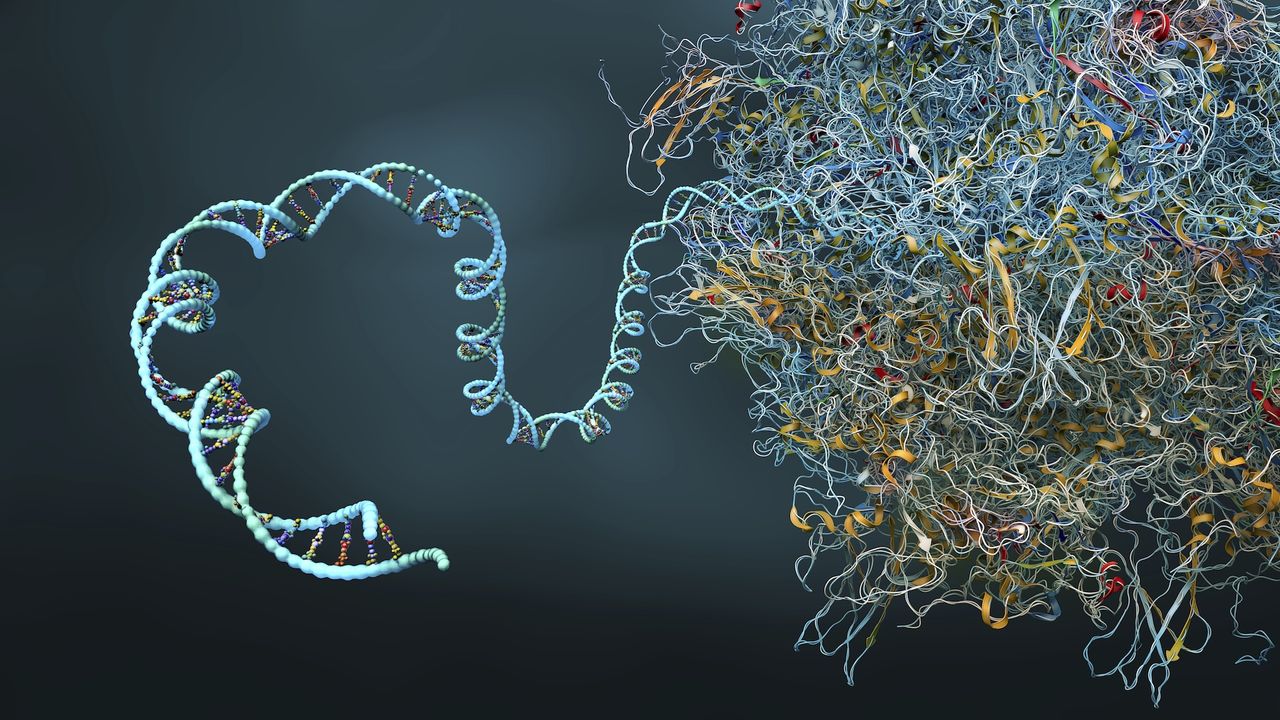
IBM and Moderna researchers successfully used a quantum simulation algorithm to predict the structure of complex secondary proteins of a 60 nucleotide Mrnm sequence, the longest ever simulated on a quantum computer.
Messenger ribonucleic acid (MRNA) is a molecule that has genetic DNA genetic information to ribosomes. It directs the synthesis of proteins in cells and is used to Create effective vaccines capable of encouraging specific immune responses.
It is widely believed The fact that all the information required for a protein to adopt correct three -dimensional conformation is provided by its sequence of amino acids or “folding”.
Although it is composed only one strand of amino acids, mRNA has a secondary protein structure composed of a series of folds which provide a specific 3D shape of a given molecule. The number of possible folding permutations increases exponentially with each nucleotide added. This makes the challenge of predicting the shape that a mRNA molecule will take insoluble at higher scales.
The IBM and Moderna experience, described in a study Published for the first time for the International Conference of IEEE 2024 on quantum IT and Engineering, has demonstrated how quantum IT can be used to increase traditional methods to make such predictions. Traditionally, these predictions generally invoked on Binary and classic computers and artificial intelligence (Ai) models such as Google Deepmind alphafold.
In relation: The AI program of Deepmind Alphafold3 can predict the structure of each protein in the universe – and show how they work
According to a new study published on May 9 on pre -impression arxiv The database, algorithms capable of operating on these conventional architectures can treat mRNA sequences with “hundreds or thousands of nucleotides”, but only by excluding higher complexity characteristics such as “pseudoknots”.
The pseudoknots are twists and turns and complex forms in the secondary structure of a molecule which are able to engage More complex internal interactions that ordinary folds. Thanks to their exclusion, the potential precision of any protein folding prediction model is fundamentally limited.
Understanding and predicting even the smallest details of the protein folds of an mRNA molecule is intrinsic to develop stronger predictions and, therefore, more effective mRNA vaccines.
Scientists hope to overcome the limits inherent in Most powerful supercomputers And AI models by increasing experiences with quantum technology. The researchers conducted several experiences using quantum simulation algorithms that leaned on quit – The quantum equivalent of a computer bit – to model molecules.
Initially using only 80 qubits (on a possible 156) on the Heron R2 Quantum processing unit (QPU), the team used a quantum variational value algorithm of risk -based conditional value (VQA based on CVAR) – a quantum optimization algorithm modeled after certain techniques used to analyze complex interactions such as Avoidance of collisions And financial risk assessment techniques – to predict the secondary protein structure of a 60 nucleotides mRNA sequence.
The best previous for a quantum -based simulation model, According to the studywas a sequence of 42 nucleotides. Researchers also put the experience by applying by applying Recent errors correction techniques To manage the noise generated by quantum functions.
In the new preparation study, the team has temporarily demonstrated the effectiveness of the experimental paradigm in the execution of simulated instances with up to 156 qubits for mRNA sequences of up to 60 nucleotides. They also carried out preliminary research demonstrating the location potential up to 354 qubits for the same algorithms in noise -free contexts.
In appearance, the increase in the number of qubits used to execute the algorithm, while increasing algorithms for additional sub-programs, should lead to more precise simulations and the ability to predict longer sequences, they said.
However, they have noted that “these methods require the development of advanced techniques to integrate these circuits specific to the problem in the existing quantum material”, indicating that better algorithms and treatment architectures will be necessary to advance research.




