Where do atoms come from? A physicist explains.

Curious children is a series for children of all ages. If you have a question you would like an expert to answer, send it to Curiiouskidsus@theconversation.com.
How are atoms formed? —Joshua, 7, Shoreview, Minnesota
Richard FeynmanA famous theoretical physicist who won the Nobel Prize, says it If he could only transmit one scientific information to future generations, it would be that everything was done atoms.
Understanding how the form of atoms is a fundamental and important question, because they constitute everything with the mass.
The question of where the atoms comes from requires a lot of physics to respond completely – and even then, physicist Have good assumptions to explain how certain atoms are formed.
What is an atom?
An atom consists of a heavy center, called the nucleus, made of particles called protons and neutrons. An atom has lighter particles called electrons that you can consider as an orbit around the nucleus.
The electrons each carry a negative load unit, Protons Everyone has a positive load unit, and neutrons have no fees. An atom has the same number of protons as electrons, so it is neutral – it has no overall load.
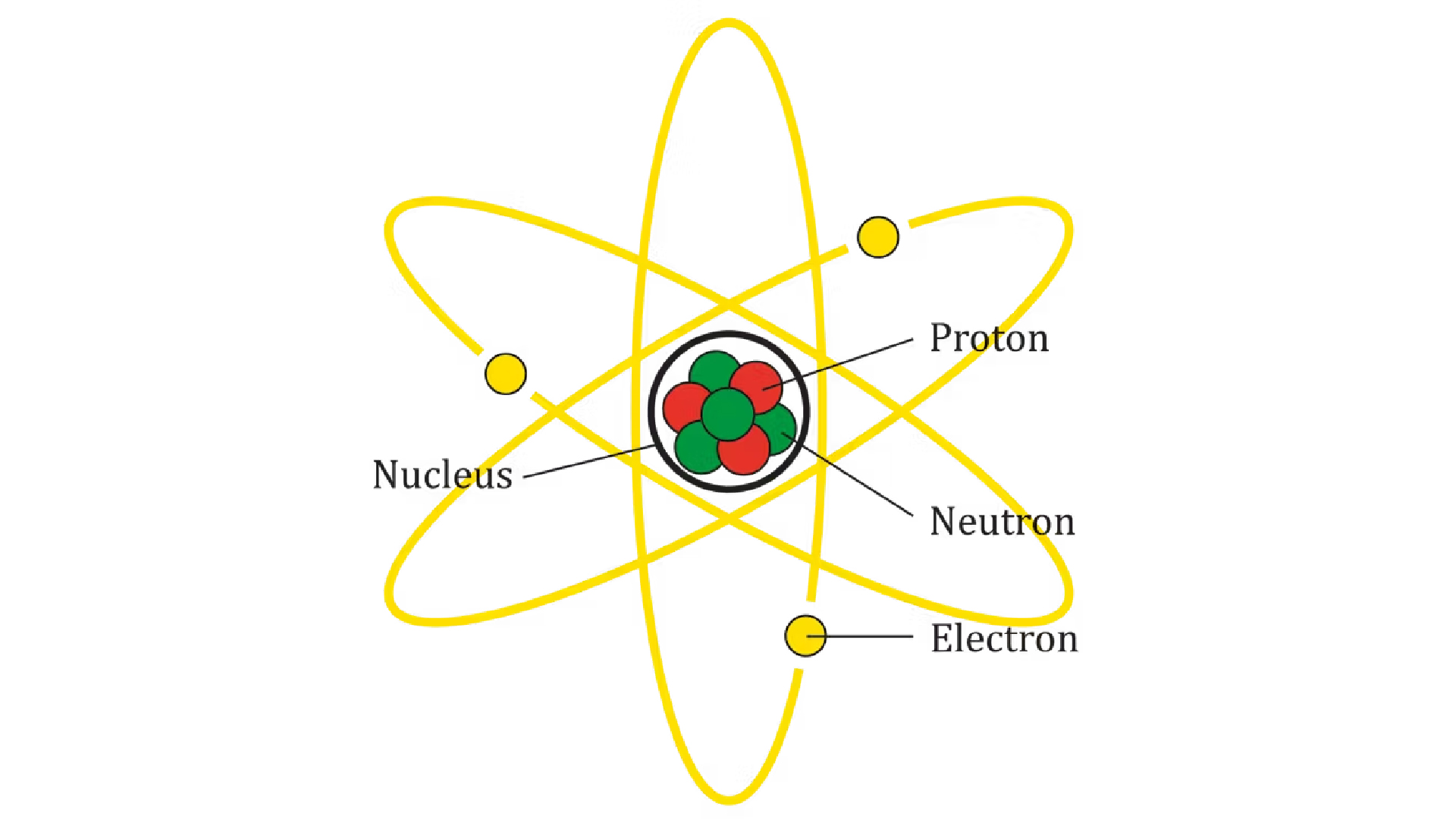
Now, most atoms in the universe are the two simplest types: hydrogen, which has a proton, zero neutrons and an electron; and helium, which has two protons, two neutrons and two electrons. Of course, on earth, there are many atoms in addition to those who are just as common, as carbon And oxygen, but I’m going to talk about it soon.
An element is what scientists call a group of atoms that are all the same, because they all have the same number of protons.
When did the first atoms formed?
Most hydrogen and helium atoms in the universe have formed around 400,000 years later Big BangWho is the name of the moment when scientists think that the universe began, about 14 billion years ago.
Why did they formed at that time? Astronomers know observation Distant explosive stars that the size of the universe was become bigger Since Big Bang. When hydrogen and helium atoms formed for the first time, the universe was on 1,000 times smaller that now.
And on the basis of their understanding of physics, scientists believe that the universe was much warmer when it was smaller.
Before this period, the electrons had too much energy to settle in the orbits around the hydrogen and helium nuclei. Thus, hydrogen and helium atoms could only form once the universe has cooled with something like 5,000 degrees Fahrenheit (2,760 degrees Celsius). For historical reasons, This process is informed of recombination – The combination would be more descriptive.
Helium and deuterium – a heavier hydrogen shape – The kernels formed even earlier, just a few minutes after the Big Bang, when the temperature was greater than 1 billion F (556 million C). Protons and neutrons can collide and form nuclei like these only at very high temperatures.
Scientists believe that almost all ordinary materials in the universe are made of around 90% hydrogen atoms and 8% helium atoms.

How are more massive atoms formed?
Thus, hydrogen and helium atoms were formed during recombination, when the colder temperature allowed the electrons to fall into the orbits. But you, me and almost everything on earth is made of many more massive atoms than hydrogen and helium. How were these atoms made?
The surprising answer is that more Massive atoms are made of stars. Make atoms with several protons and neutrons glued together in the nucleus requires the type of high energy collisions that occur In very hot places. The energy necessary to form a heavier nucleus must be large enough to overcome the repulsive electrical force that the positive loads, like two protons, feel with each other.
Protons and neutrons also have another property – a bit like a different type of load – which is strong enough to bind them together once they can get closer very close. This property is called the strong forceAnd the process that brings together these particles is called fusion.
Scientists think that most elements of the carbon to the iron merged into the stars heavier than our sun, where the temperature can exceed 1 billion F (556 million C) – the same temperature as the universe was when it had only a few minutes.
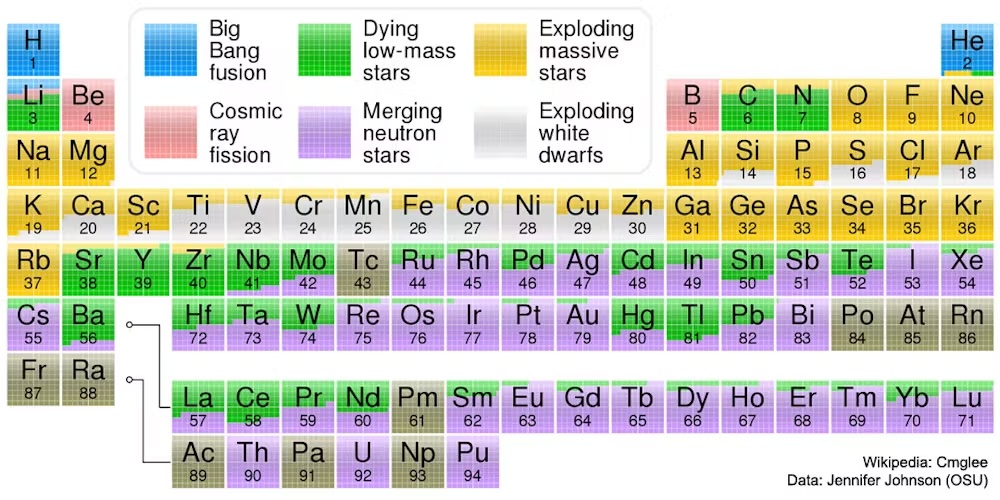
But even in hot stars, elements heavier than iron and nickel will not form. These require additional energy, as heavier elements can more easily break into pieces.
In a dramatic event called a supernovaThe inside nucleus of a heavy star suddenly collapses after he does not lack fuel to burn. During the powerful explosion, this collapse is triggered, heavier elements that iron can form and be ejected into the universe.
Astronomers always find the details of other fantastic stellar events that form larger atoms. For example, collided neutron stars can free up huge amounts of energy – and Elements such as gold – On the way to black holes.
Understanding the way atoms are manufactured simply require learning a little general relativity, as well as nuclear physics, particles and atomic physics. But to complicate things, there are other things in the universe that does not seem to be made at all from normal atoms, called dark matter. Scientists are investigate what dark matter is And how it might be formed.
This published article is republished from The conversation Under a creative communs license. Read it original article.



