For kids with Barth syndrome, time is running out, parents say, unless the FDA acts

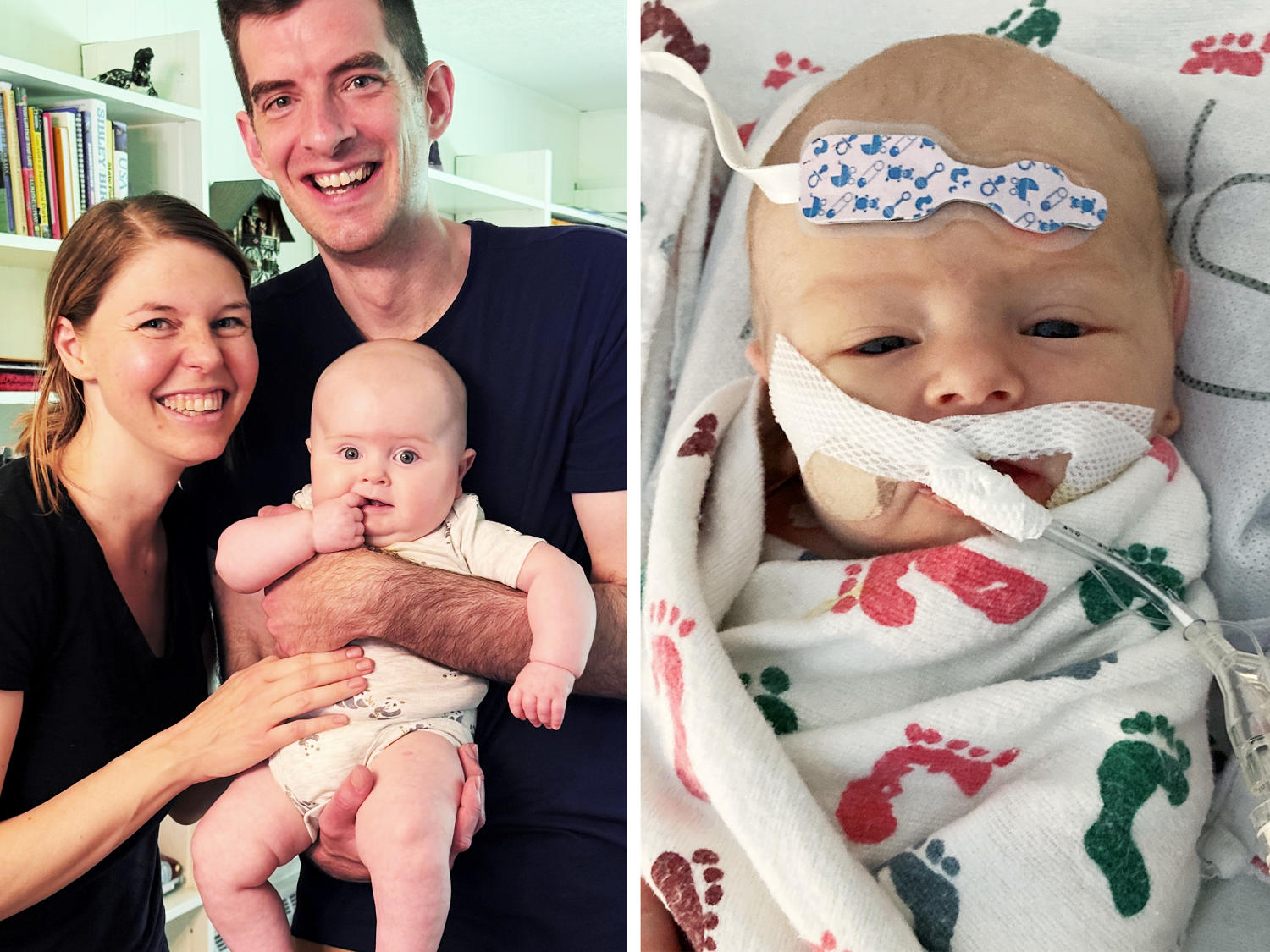
Gilbert Dryden probably only has enough medication to make him pass at the end of October, his mother, Madison, figures.
Gilbert, seven months, has a rare genetic disease called Barth syndrome, a condition that can have disastrous consequences, such as heart failure, extreme muscle weakness and considerably reduced life expectancy.
Children who die early often do not see their fifth anniversary. According to the foundation of Barth syndrome.
What kept Gilbert alive and works relatively well, according to his family, is an experimental medication called Elamipretide, manufactured by stealth biotherapeutics. Small studies have suggested that it be safe and effective in treating ultra-rare disease. In the United States, only 150 people have the condition.
“Our children die. We have seen that this medication is working, “said Kate McCurdy, co -founder of the Barth Syndrome Foundation. “This drug completely saves the lives of babies.”
After a process of more than a decade to put the drug on the market, the approval of the treatment has repeatedly struck road dams with the Food and Drug Administration.
A reverse occurred in the spring, when an Inspection of the FDA found problems in a stealth manufacturing plant. The problems were not made public, but were apparently not obviously obvious to justify a regulatory action. The company said the problems had been solved.
At the end of May, after a consulting committee voted earlier (in October 2024) to recommend therapy, the FDA refused to approve it. The agency did not reveal why.
An ultra-rare disease such as Barth syndrome with an ultra-small population of patients who can be tested for clinical efficiency faces obstacles to FDA drug approval. There are not enough patients to do robust randomized clinical trials, said McCurdy.
“It is practically impossible to conduct tests that give data that is conclusive beyond reasonable doubt. Statistically, you just can’t do it, “she said.
A few weeks later and after a meeting between Needham, Stealth based in Massachusetts and the FDA at the end of June, the real bomb came: the company said that the agency had informed the stealth on August 4 that it should submit a new medication request again – for the third time.
It could use a special route called “accelerated approval” in this submission, but the manufacturing problem would extend the calendar to examine the elamipretide for at least six additional months, according to the company.
Stealth said no additional clinical data or additional security data has been requested. But without any course correction in the process, private company could lack money.
The delay is devastating for families like the Drydens.
“When we heard this news, we immediately went to the refrigerator to count the number of bottles we stayed, because it is how long we have this medication. It is the only guarantee that we have at the moment that Gilbert will not die,” said Madison Dryden, 35, from Aurora, Colorado. “It’s the highest level of despair.”
Stealth announced on Monday that he submitted that he had submitted his third request for approval from the Élamipretide – asking for an accelerated track under a timeline much tighter than the agency initially recommended.
What is Barth syndrome?
Madison Dryden and her husband, Andrew, were not sure of what was wrong with Gilbert in the hours and days after his birth on Christmas Eve in 2024.
“His heart function was so low. He was so sick, his body and his feet were purple, his hands were purple,” said Dryden. “He couldn’t eat.”
Gilbert was admitted to the neonatal intensive care unit and received rescue care while doctors urgently tried to find a diagnosis.
In a few days, said Dryden, Gilbert was transported by plane to the Colorado children’s hospital with a heart that had difficulty pumping blood. The underlying cause was quickly revealed: Barth syndrome.
The disease alters the mitochondria of cells, which are a bit like tiny batteries that generate energy so that cells work properly.
Chromosomal disorder almost exclusively affects boys. About 85% of early deaths occur before the age of 5. Those who survive longer receive heart medication and may need cardiac transplantation.
Children with Barth syndrome often count on drugs such as beta-blockers and ECA inhibitors to keep their hearts that are as well as possible.
Gilbert was lucky, at least at the start. He found a life buoy thanks to access to the treatment of stealth. The elamipretide, a daily injection, works by helping to repair damaged mitochondria.
In clinical trials, patients have shown an improvement of 45% of muscle strength and an improvement of 40% of the heart function, depending on the medication. Most participants have remained safely on the medication for over eight years.
In October 2024, an FDA advisory committee voted 10-6 in support of therapy, paving the way to the agency to erase the first medication to treat Barth syndrome. The FDA is not required to follow the directives of the Advisory Committee, but it almost always does.
A spokesman for the Ministry of Health and Social Services, who oversees the FDA, wrote in an email that the “FDA has carefully examined the advice of the members of the Advisory Committee, including their justification for their vote. Although there is often a rate of compliance with the FDA and advisory advice recommendations, “said the spokesperson,” there is not always a concordance “.
“Convincing medical needs” for approval
After the recommendation of the Advisory Committee, the rejection of the FDA was unexpected.
“This is a small sample size,” said a former FDA official who was involved in the drug approval process. The person asked not to be identified in order to speak freely.
But the former official quickly pointed out that, given the recommendation of the Advisory Committee for Approval, the “incredibly convincing medical need” of those who live with Barth syndrome and the small sample of people who seemed to have benefited from the treatment, it certainly seemed that the elamipretide was going to pass through the finish line.
A lack of continuity in leadership, however, may have added to the already difficult challenge to guide treatment through the final hedges, said the former official.
The FDA has seen many senior officials leaving the agency in recent months.
“Those who have taken over are not as familiar, or perhaps it would be more appropriate to say that they are inexperienced in the way we make drug approvals, how we get things done, and they are also a little afraid of the current environment,” said the former official. “And I think that has led to an unfavorable result for some of these rare products.”
The HHS spokesman did not immediately respond to a request for comments on the rejection by the agency of drug or leadership problems.
An editorial of the Wall Street Journal recently offered a scathing assessment of what is happening at the FDA, wondering if the agency and the commissioner Marty Makary to accelerate drugs saving lives corresponds to the reality of their actions.
The newspaper said there were averages of 52 annual drug approvals under the first Trump administration and 48 under President Joe Biden, but “there were only 22 in the first seven months of this year”, projecting only 38 for the year.
Elamipretide was quoted in the newspaper’s play as a victim of the current environment.
The former FDA official stressed that the treatment had also reached stumbling blocks under the previous administration, but it was heading for the right direction.
“Now it looks like a hot potato that has just been thrown,” said the former official. “And it’s not fair. It’s just not good.”
Families left with some alternatives
The Drydens say that the FDA decision has removed the carpet below – and many other families.
Dr. Kathryn Chatfield, doctor of Gilbert and specialist in cardiology and pediatric genetic at the Children’s Hospital in Colorado, said that the inaction of the FDA leaves families with few alternatives.
“We just don’t know what’s going to happen,” said Chatfield. “We will have to look at them very closely because they are at risk of recurrence of heart failure and rehospitalization and potentially decompensation to the point where they must live in the hospital until they can obtain a heart transplant.”
A transplant tackles the heart problem but did not led to continuous muscle and skeletal problems.
“I cannot agree to sacrifice my child’s life for a bureaucratic process,” said Madison Dryden.
The last obstacle to approval has families and defenders of Barth syndrome, including certain members of the Congress, looking for more information and responses.
“Time is gasoline here, and we have to bring these drugs to these patients as soon as possible,” said the representative Buddy Carter, R-GA., Member of the energy and health subcommittee on health.
Carter spoke to NBC News a few days before the FDA asked for a submission in early August and said that he had sent a letter to the agency requesting “clarity” on the elamipretide.
“There are not many options there,” he said. “In fact, for Barth syndrome, this elami -retis is really the only medication we know that works.”
Carter said there were six families in Georgia alone with whom he was in contact with whom has a loved one with rare disease.
He hopes that an “accelerated path” request could prove a viable option for families and for the company to continue his research.
After the recent FDA decision, Carter told NBC News in a statement that he was “disappointed” from the recommendation “now that all problems seem solved”.
Carter said he continued to put pressure on the FDA and that “patients will undergo other delays”.
He also recognized that the economy of a private company, such as stealth, trying to continue to develop a drug for such a small population of patients would be extremely difficult without the support of the FDA.
He said he felt deeply for families in the midst of a terrifying test.
“I would call everyone that I could (too), making sure that my child or my grandchild had this medication,” he said in an interview.
Madison Dryden and her husband find themselves with what they call a “giant unknown” in the absence of approval, struggling for an explanation of their daughters aged 7 and 3, because families are in a race to keep beings dear alive.
“They know that someone does not give permission to Gilbert to have his medicine, and that he continues to say no, and it is our children-as if they could not lose … We cannot make them lose their little brother,” she said.



